In this, the final exploration before the dreaded final assignment, we took on the very challenging task of boiling water, measuring its temperature, and graphing the data. You would think that for two people with a combined total of 33 years of education, that this would be a no brainer. You thought wrong!
This is not to say that we were unable to boil water. We had a cookbook, and used my mother's recipe to perfection. (Take one pot of water, put it on the stove, and heat until boiling. Feel free to use this the next time you need to create boiling water for any household usage.) But, when we took our data and matched it against the formula for Newton's Law of Cooling, we found that we had not done a good job of measuring the temperature. Let me take you back to the beginning...
It was a fine Autumn day. The temperature inside Lauren's house was balmy 74 degrees Fahrenheit. We proceeded to bring a pot of water to boil, and to measure the temperature at one minute intervals. Here is that collected data
| Minute | Temperature |
| 0 | 212 |
| 1 | 204 |
| 2 | 192 |
| 3 | 186 |
| 4 | 180 |
| 5 | 175 |
| 6 | 171 |
| 7 | 168 |
| 8 | 164 |
| 9 | 162 |
| 10 | 159 |
| 11 | 155 |
| 12 | 153 |
| 13 | 151 |
| 14 | 149 |
| 15 | 147 |
| 16 | 145 |
| 17 | 143 |
| 18 | 142 |
| 19 | 139 |
| 20 | 139 |
| 21 | 136 |
| 22 | 135 |
| 23 | 134 |
| 24 | 132 |
| 25 | 131 |
| 26 | 130 |
| 27 | 129 |
| 28 | 128 |
| 29 | 127 |
| 30 | 125 |
And, upon our return to 6680 class, we looked up the formula for Newton's Law of Cooling to help us model the actual cooling function that our data should obey. The formula for Newton's Law of Cooling is:
Where t = time
in minutes, ![]() is the room temperature,
is the room temperature, ![]() is the original temperature, in this case,
212 degrees Fahrenheit, and
is the original temperature, in this case,
212 degrees Fahrenheit, and ![]() contains
k, the experimental constant. In order to find k, we first solved
the equation for k given the following values of t: T(3), T(11),
T(19), T(26), and T(29). Then, we averaged these values to find
our value for k. We calculated k to be approximately .042901.
Then, armed with this knowledge, we set up our Excel file to calculate
the true cooling function or each data point based on Newton's
Law.
contains
k, the experimental constant. In order to find k, we first solved
the equation for k given the following values of t: T(3), T(11),
T(19), T(26), and T(29). Then, we averaged these values to find
our value for k. We calculated k to be approximately .042901.
Then, armed with this knowledge, we set up our Excel file to calculate
the true cooling function or each data point based on Newton's
Law.
| Minute | Temperature |
|
| 0 | 212 | 212 |
| 1 | 204 | 206.204859462488 |
| 2 | 192 | 200.653078735481 |
| 3 | 186 | 195.334438222578 |
| 4 | 180 | 190.239147486782 |
| 5 | 175 | 185.357827228474 |
| 6 | 171 | 180.681492020207 |
| 7 | 168 | 176.201533766522 |
| 8 | 164 | 171.909705858361 |
| 9 | 162 | 167.798107992885 |
| 10 | 159 | 163.859171630773 |
| 11 | 155 | 160.085646064217 |
| 12 | 153 | 156.470585069981 |
| 13 | 151 | 153.007334122942 |
| 14 | 149 |
|
| 15 | 147 | 146.511029777924 |
| 16 | 145 | 143.46601812515 |
| 17 | 143 | 140.548877997494 |
| 18 | 142 | 137.754239587282 |
| 19 | 139 | 135.076958585322 |
| 20 | 139 | 132.512106711368 |
| 21 | 136 | 130.05496264225 |
| 22 | 135 | 127.701003320969 |
| 23 | 134 | 125.445895630749 |
| 24 | 132 | 123.285488418732 |
| 25 | 131 | 121.215804854627 |
| 26 | 130 | 119.233035110248 |
| 27 | 129 | 117.333529346465 |
| 28 | 128 | 115.51379099466 |
| 29 | 127 | 113.77047032032 |
| 30 | 125 | 112.10035825692 |
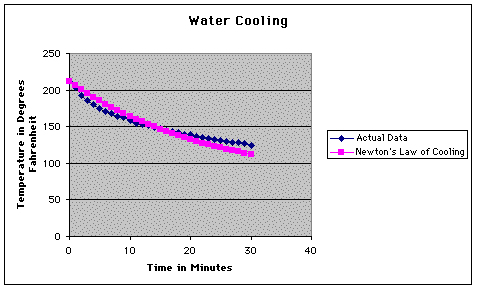
As you can see, we didn't do a very good job of measuring the temperatures. Our perceived fears regarding this were justified when we calculated the average error of our calculations.
In order to find our average error, we took the difference between our measured temperature an the calculated one, and squared it. We then averaged the errors to find our average error.
| Minute | Temperature | Newton's Data | Error |
| 0 | 212 | 212 | 0 |
| 1 | 204 | 206.204859462488 | 4.86140524932501 |
| 2 | 192 | 200.653078735481 | 74.8757716024274 |
| 3 | 186 | 195.334438222578 | 87.1317369311177 |
| 4 | 180 | 190.239147486782 | 104.840141256067 |
| 5 | 175 | 185.357827228474 | 107.28458489492 |
| 6 | 171 | 180.681492020207 | 93.7312877373225 |
| 7 | 168 | 176.201533766522 | 67.265156123395 |
| 8 | 164 | 171.909705858361 | 62.5634467657846 |
| 9 | 162 | 167.798107992885 | 33.6180562971587 |
| 10 | 159 | 163.859171630773 | 23.6115489373111 |
| 11 | 155 | 160.085646064217 | 25.8637958904902 |
| 12 | 153 | 156.470585069981 | 12.0449607279755 |
| 13 | 151 | 153.007334122942 | 4.02939028112886 |
| 14 | 149 | 149.68951814659 | 0.475435274477404 |
| 15 | 147 | 146.511029777924 | 0.239091878077475 |
| 16 | 145 | 143.46601812515 | 2.35310039236828 |
| 17 | 143 | 140.548877997494 | 6.0079990711704 |
| 18 | 142 | 137.754239587282 | 18.0264814822011 |
| 19 | 139 | 135.076958585322 | 15.3902539412768 |
| 20 | 139 | 132.512106711368 | 42.092759324679 |
| 21 | 136 | 130.05496264225 | 35.3434691850422 |
| 22 | 135 | 127.701003320969 | 53.2753525205067 |
| 23 | 134 | 125.445895630749 | 73.1727015600435 |
| 24 | 132 | 123.285488418732 | 75.942712100058 |
| 25 | 131 | 121.215804854627 | 95.7304746427387 |
| 26 | 130 | 119.233035110248 | 115.927532937147 |
| 27 | 129 | 117.333529346465 | 136.106537509792 |
| 28 | 128 | 115.51379099466 | 155.905415325043 |
| 29 | 127 | 113.77047032032 | 175.020455545546 |
| 30 | 125 | 112.10035825692 | 166.40075709982 |
| 60.2945745962713 |
As you can see, our average error was over 60. Not too good. There are many possibilities as to why this happened. One major cause of error could be in the accuracy of measurement of our thermometer. Another reason of course is human error in reading the thermometer. (I was in charge of this, so you know it definitely has errors.) I guess we could repeat the experiment and try to do a better job of measuring the data, but it's the end of the semester, and I'm not really keen on spending another half-hour of my life watching water cool down. Perhaps over Winter Break...